The spectrum
All methods of optical spectroscopy have in common that absorption and emission are based on the quantum physical nature of light, i.e. the spectra obtained show discrete energy levels of electronic excitation or vibrational excitation. All observed energy levels depend on the type of atoms involved, on the type of bonds between them and the surrounding neighboring atoms. Therefore, the structure or composition can be deduced from the energies that are absorbed from radiation or emitted as radiation.3
In the spectrum, these energies or energy levels can be found as 'bands'4. Contrary to what is expected with discrete energy levels, the bands are generally not depicted as a 'sharp' line, but are broadened into a band due to thermal excitation (homogeneous broadening). As a measure for the intensity of a band or for the quantization, the integral band area is used instead of the amplitude in the spectrum. The full-width at half maximum (FWHM) υ1/2 is also used to characterize the bands.
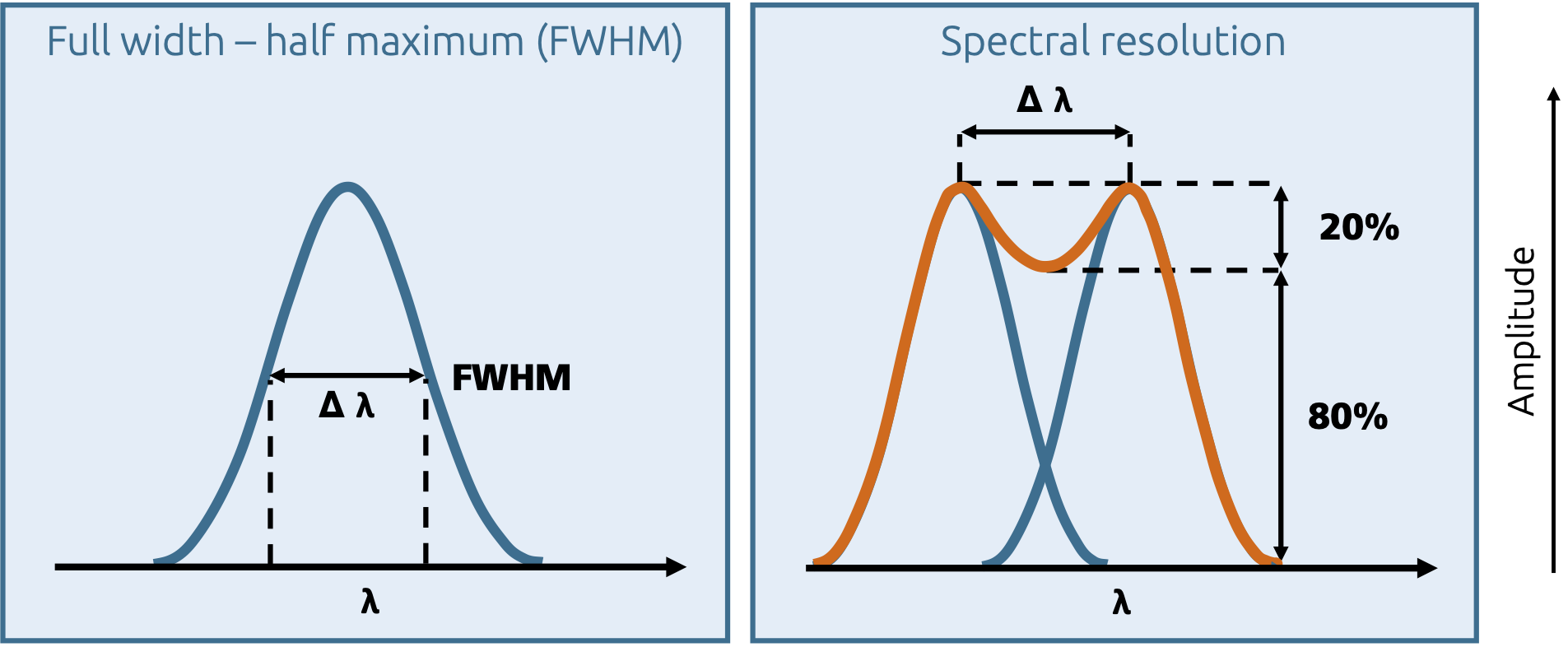
Full width, half maximum (FWHM) and resolution of bands
Bands can also form in the spectrum if individual absorptions cannot be separated due to a lack of resolution in the spectrometer (inhomogeneous broadening). In addition, individual absorptions can also overlap in the spectrum and form a band, but then mathematical methods can be used to separate the bands in order to still obtain a quantitative statement. This can often be recognized by so-called 'shoulders' (step-shaped sections) in such integral bands. In the spectrum, two bands are considered resolved if the intensity between their maxima falls to 80 % or less.
References:
3 W. E. Steger, „Strukturanalytik“, Dt. Verlag für Grundstoffindustrie, 1. Auflage 1992, ISBN 3-342-00044-9
4 The word 'band' is an artificial word and originally referred to non-resolvable rotational absorption in gas spectra.
