Interaction of light and matter
Matter can reflect or absorb electromagnetic radiation or the radiation can pass through the matter with little or no interaction. In the latter case, this is referred to as transmission.
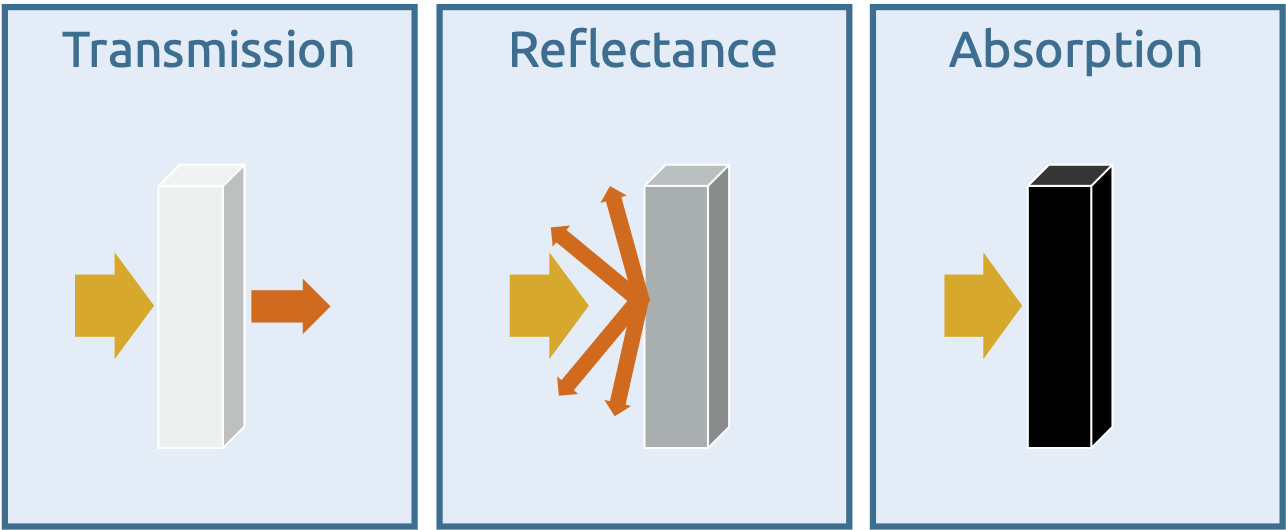
The three basic principles of the interaction of light and matter
If matter completely absorbs the radiation, optical spectroscopy (UV, VIS, IR) is not possible. All radiation energy then remains in the matter and is re-emitted in other areas of electromagnetic radiation. Such bodies are also known as 'black bodies', an illustrative example being black surfaces in VIS spectroscopy.
Special cases are fluorescence spectroscopy and RAMAN spectroscopy, both of which actively utilize the absorption of the excitation radiation and measure the (re-)emission of the slightly wavelength-shifted radiation. However, the mechanisms behind this are completely different in each case.2
Depending on the spectral range, the electromagnetic radiation interacts very differently with the particles under investigation. As the generic terms 'electron excitation spectroscopy' and 'vibrational spectroscopy' suggest, in one case the outer electrons are excited (UV, VIS, fluo) and in the other case the intermolecular vibrations between the atoms (IR, RAMAN).
References:
2 In fluorescence spectroscopy, the change in wavelength is caused by radiationless relaxation of the excited electrons, whereas in RAMAN spectroscopy the change in wavelength is caused by inelastic scattering of the radiation on the atoms, with excitation of molecular vibrations.
